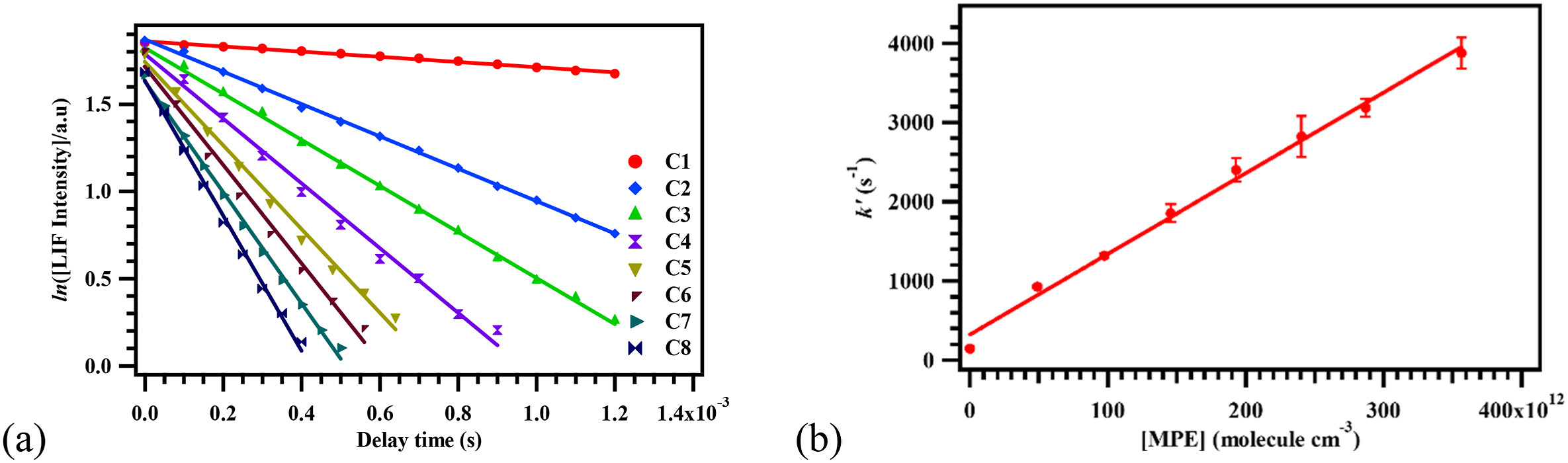
Oxygenated volatile organic compounds (OVOCs) are emitted into the atmosphere via biological processes, oxidation of hydrocarbons, evaporation of oxygenated solvents or fuels and incomplete combustion of hydrocarbon-fueled sources. In the troposphere, the degradation mechanisms of these VOCs are triggered by oxidising agents like Cl atoms and OH radicals. In the present work, the kinetics were investigated for the reactions of methyl n-propyl ether (MPE) with OH radicals and Cl atoms using pulsed laser photolysis-laser induced fluorescence and relative rate techniques, respectively, in the range of 278–363 K. No significant temperature-dependence was observed for the reaction of MPE with OH radicals, whereas a slight negative temperature-dependence was asserted in the Cl reaction. The rate coefficients were also determined computationally at the CCSD(T)/aug-cc-pVDZ//M06-2X/6-311+G(d,p) and CCSD(T)/6-31+G(d,p)//BHandHLYP/6-31+G(d,p) level of theories over 250–400 K for the OH- and Cl-initiated reactions of MPE, respectively. The Arrhenius expressions for the title reactions determined are: cm3 molecule−1 s−1 and cm3 molecule−1 s−1. The rate coefficients were estimated using SAR and compared to the experimentally and computationally determined kinetic data. The products formed in the reactions were detected using the GC-MS. The atmospheric lifetimes of MPE were estimated, accounting for the rate coefficients of both reactions. The impacts of MPE emissions on the atmosphere are discussed using important atmospheric parameters.
-
Call
-
E-mail
Journal Details
1. OH radical and Cl atom initiated Reactions of Methyl n-propyl ether: Kinetics and Atmospheric Implications.
B. Kar and B. Rajakumar Atmos. Environ., , 120717, 336