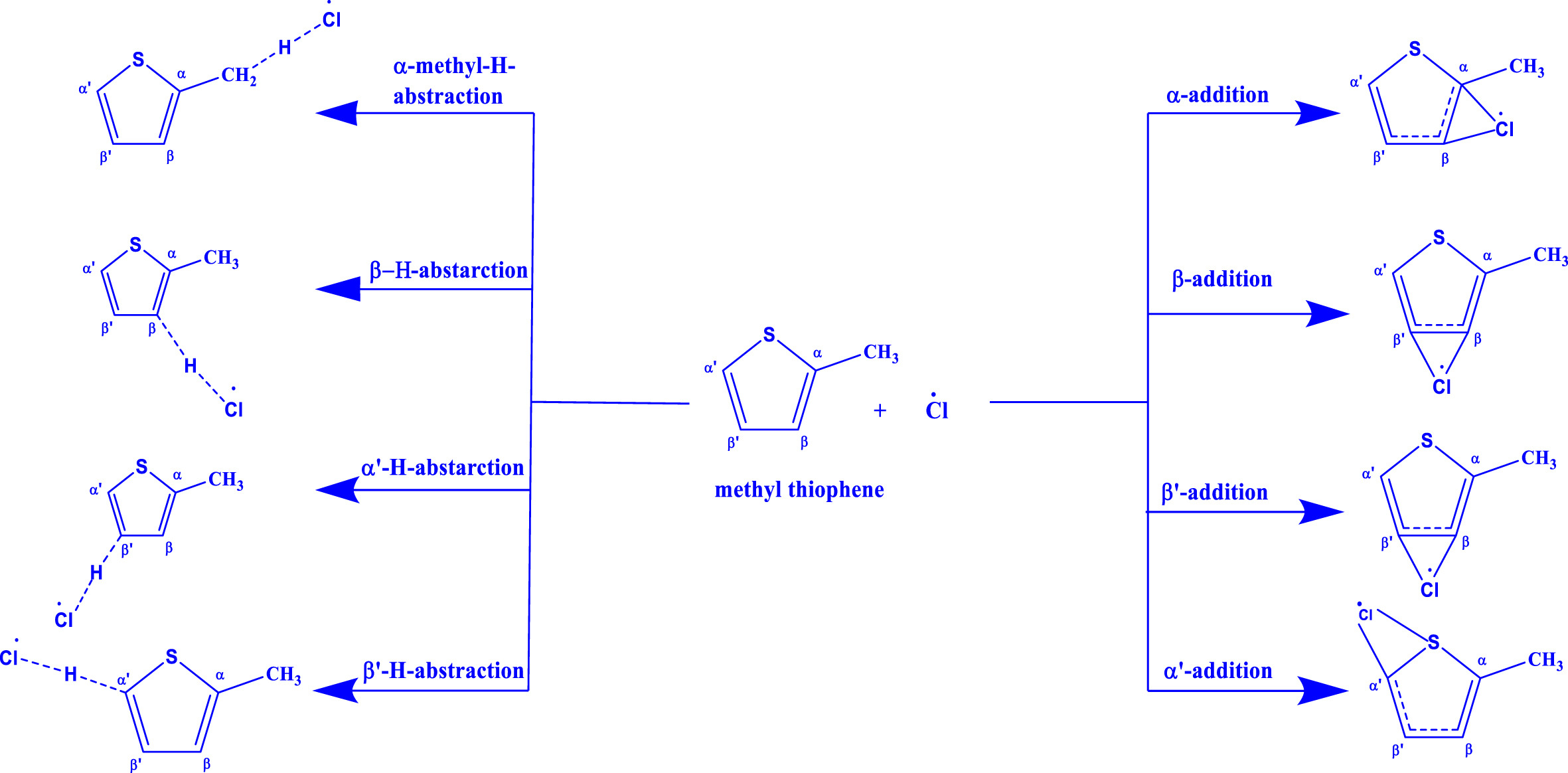
The rate coefficient for the reaction of methyl thiophene (MeTp) with Cl atom was measured to be (1.09 ± 0.24) ×10-10 cm3 molecule-1s-1 at 298K and 760 Torr using the relative rate (RR) method. The experimental temperature-dependent rate coefficient was measured to be cm3 molecule-1 s-1. The theoretical rate coefficients for the reaction of MeTp with Cl-atom and OH radical were calculated at BD(T)/VDZ//BHandHLYP/6-31+g* and BD(T)/AVDZ//M06-2X/6-311++g** level of theory respectively. The Arrhenius equation from the theoretically calculated rate coefficients for MeTp + Cl using the MESMER simulation was obtained to be cm3 molecule-1 s-1. The experimental and theoretical rate coefficients show a negative temperature dependence behaviour over the studied temperature range. The rate coefficient for MeTP + OH was calculated as cm3 molecule-1 s-1 from CVT/SCT methods. Following the MESMER simulation for MeTp+OH reaction, the Arrhenius expression was found to be cm3 molecule-1 s-1. At 298 K, the rate coefficient was calculated to be 5.60 × 10-11 cm3 molecule-1 s-1 and 6.23 × 10-11 cm3 molecule-1 s-1 for the reaction of MeTp with OH from CVT/SCT method and MESMER simulations. Both calculation methods demonstrated a negative temperature dependency over the temperature range of 200-400 K. No pressure dependency was observed over the 5-760 Torr in the MESMER calculations. The atmospheric lifespan of MeTp was estimated to be ∼100 days in ambient conditions and ∼1 day in polluted or coastal areas.
-
Call
-
E-mail
Journal Details
1. A Comprehensive Gas-phase Kinetics of α-Methyl Thiophene with Cl-atom and OH-radical: Experimental and Theoretical Studies.
Prasanna Kumar Bej and B. Rajakumar Atmos. Environ., 2025, PP.121078,